DOT Oral Fluid Specimen Collector Training and Certification Course
|
COMING SOON |
 |
| DOT Oral
Fluid Specimen Collector Online Training
Includes the Procedures and Proficiency Demonstration by webcam (Mocks with the Instructor) and the Certificate for compliance Get on the list to be notified for training |
|
|
Oral Fluid Specimen Collector Instructor
Train the Trainer Includes the Procedures and Proficiency Demonstration by webcam (Mocks with the Instructor) and the Certificate for compliance Get on the list to be notified for training |
|
|
When this Course comes available - Hartman Training Center will be offering the training certification for the collection of oral (saliva) fluid specimens for Federal drug testing. Note: to date, the Department of Transportation Rules do not allow oral fluid specimen collections for drug testing. ONLY urine specimens are allowed under the 49 CFR Part 40 regulations. The new 2020 Federal Drug Testing Custody and Control Form contains a checkbox for "ORAL FLUID", you may not begin collecting oral fluid specimens from donors until the Office of Drug and Alcohol Policy and Compliance develops the mandatory guidelines for oral fluid testing and the Substance Abuse and Mental Health Services Association (SAMHSA) and the Department of Health and Human Services (HHS) determine the requirements for saliva testing devices and laboratory protocols.
Once these steps are completed, the Federal Drug Administration (FDA) must approve the work done in this area by the SAMHSA and the HHS. After all this happens, the DOT needs to establish the new Guidelines for oral fluid specimen collection. Once the Department of Transportation Rules are in place regarding (saliva) Oral Fluid Collections we will be offering the new training course and certification for (saliva) Oral Fluid Specimen Collectors. |
|
In addition to the changes regarding oral fluids, the revised CCF also includes the following changes: •Copies 1-5, Step 1: Added “CDL State and No.” to donor identification (FMCSA only); •Copies 1-5, Step 1: Added “Other” (i.e., e-mail) to Collector Contact Info; •Copy 1, Step 5a: Removed analytic names and checkboxes; repositioned results and checkboxes; and added a line for the certifying scientist to record the positive analytics and concentrations if a positive result is recorded. •Copies 2-5, Step 5: Added a line for the donor e-mail address. •Copy 5: Removed instructions for completing the CCF from the back of the form. Instructions for completing the CCF
Instructions for Completing the Federal Drug Testing Custody and Control Form for Oral Fluid Specimen Collection
When making entries on a paper CCF, use black or blue ink pen and press firmly Collector ensures that the name and address of the HHS-certified laboratory are on the top of the Federal CCF and the Specimen Identification (I.D.) number on the top of the Federal CCF matches the Specimen I.D. number on the labels/seals.
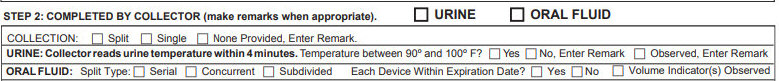
• Collector ensures that the required information is in STEP 1. Collector enters a remark in STEP 2 if Donor refuses to provide his/her SSN, Employee I.D., or CDL state and number. • Collector marks the oral fluid box above STEP 2. • Collector notes any unusual behavior or appearance of Donor in the Remarks line in STEP 2. If the Donor’s conduct at any time during the collection process clearly indicates an attempt to tamper with the specimen, Collector notes the conduct in the Remarks line in STEP 2 and takes action as required. STEP 2: • Collector checks the Split or Single Collection box in STEP 2. For split specimen collections, Collector checks the appropriate split type box (i.e., Serial, Concurrent, or Subdivided).
• Collector checks that each collection device is within its expiration date before use and marks the appropriate box in STEP 2. For serial split collections: Collector records times in STEP 2 when Donor removes the A specimen device from their mouth and when Donor places the B specimen device in their mouth (time between A and B collections must not exceed two minutes). If two minutes is exceeded, Collector discards the first specimen, collects the second specimen as the A specimen and collects a third specimen as the B specimen. Collector enters explanatory remarks in STEP 2. • Collector observes volume - indicators for A and B specimens during collection and marks the appropriate box in STEP 2. If the volume is less than required by the federal agency, Collector takes action as required, and enters remarks in STEP 2. • Collector inspects the specimen and notes any unusual findings (e.g., unusual color, presence of foreign objects or material) in the Remarks line in STEP 2 and takes action as required. • If no specimen is collected by the end of the collection process, Collector checks the None Provided box, enters a remark in STEP 2, discards Copy 1, and distributes remaining copies as required. STEP 3: • Donor watches Collector cap each specimen tube and affix the label/seal on each specimen tube. • Collector dates the specimen tube label(s)/seal(s) after placement on the specimen tube(s). • Donor initials the specimen tube label(s)/seal(s) after placement on the specimen tube(s). • Collector instructs Donor to read and complete the certification statement in STEP 5 on Copy 2 (signature, printed name, date, email address, phone numbers, and date of birth). If Donor refuses to sign the certification statement, Collector enters a remark in STEP 2 on Copy 1. For ECCFs: if the donor refuses to sign electronically but is willing to sign a paper CCF, Collector prints ECCF Copies 1-5. Donor signs in STEP 5: of Copies 2-5 using a wet-ink signature and Collector signs in STEP 4 of Copies 1-5 using a wet ink signature. STEP 4: Collector completes STEP 4 on Copy 1 (signature, printed name, date, time of collection, and name of delivery service) and places the sealed specimen tube(s) in a leak-proof plastic bag. Paper CCF: Collector places Copy 1 in the leak-proof plastic bag. Electronic CCF: Collector places printed copy of Copy 1 in the leak-proof plastic bag and/or places package label (with Specimen I.D., test facility name and contact information, and collection site name and contact information) on the outside of the bag. • Collector seals the bag, prepares the specimen package for shipment, and distributes the remaining CCF copies as required.
|
Alcohol Supplies for equipment Drug & Alcohol Training Evidential Breath Machines Drug Testing Kits Random Program Software Shipping
Email Us Accessibility Gloves Alcohol Screener Cal Check/Calibration Training for DER's Employees & Employers Software Updates View Cart
|
|
|
Evidential Breath Alcohol Machines |
Need a small loan to help offset the cost? We may have the answer! We offer a monthly payment plan and you can add in the training as well, choose your equipment and training needs by shopping the site to gather your total |
|
|
MASTER TRAINER |
Hartman Sales and Training Center Washington, Ga Phone: 724-388-1356 Email: thartman@ptd.net
Questions ? Call or email us